what fossil fuel is mostly used for transportation5 carat diamond ring princess cut • July 4th, 2022
what fossil fuel is mostly used for transportation
The absorption of UV radiation by ozone generates heat, which is why Fig. problems. This UV radiation can cause skin damage and lead to certain forms of cancer, including the most fatal form, melanoma. By carpooling, even with just one other person, the potential financial
For example, one atom of oxygen is 16 grams per mole and carbon is 12
Because of its properties as a powerful oxidant, ground-level O3 can have adverse effects on human health, agricultural crops, and forests. Ground-level ozone is a secondary pollutant. 1.8, O3 concentrations are small, but increase in the stratosphere (about 90% of the atmosphere's O3 lies in the layer between 10 and 17 km above the earth's surface up to an altitude of about 50 km). The Green Vehicle Guide lists pollution levels for all recent model year passenger
most fuel-efficient vehicle that meets your needs. In the data shown here, the seasonal variations and the effects of the variations in the solar flux due to the 11-year solar cycle have been removed (which would produce about a 1.21.5% variation from solar minimum to solar maximum). The rate at which light is received by the photomultiplier tube is dependent on the concentrations of O3 and ethylene. Because of this, problematic ozone levels occur most frequently on hot summer afternoons. The more ozone depletion, the larger the increase in incoming UV. In 1973, chlorine was found to catalyze ozone destruction. Harm is also caused to aquatic systems and crops. The EPA website will help
Satellite measurements began in late 1978, while a reasonably representative global network of ground-based stations has been in operation since the 1960s. However, high up in the atmosphere in a region known as the stratosphere, ozone filters out incoming radiation from the Sun in the cell-damaging ultraviolet (UV) part of the spectrum. The rate at which the
Until then, organisms had sheltered in aquatic ecosystems, because water rapidly reduces the UV-B flux. (EPA420-F-08-025), Average In-Use Emission Factors for Urban Buses and
We use some essential cookies to make this website work. In the 1970's it became apparent that marked seasonal depletion of the ozone layer was taking place over Antarctica. Its concentration in the lower troposphere (often called surface ozone) was merely about 0.02 ppmv or less before the industrial revolution. By continuing you agree to the use of cookies. Indeed, in the absence of this filtering effect, life could not exist on earth. and choose the cleanest vehicle that meets their needs. Consumers can select a vehicle model, determine
Figure 5. There are also indications that part of the increase in ozone is related to the effects of the Montreal Protocol to reduce levels of stratospheric chlorine. For example, the area where the impacts of ozone on forests have been most intensively studied is in the San Bernadino mountains, which surround the city of Los Angeles. ge2021 environmental Passenger cars and light-duty trucks also emit small amounts of
Sign up for Gov Delivery emails on many MPCA topics, EDA: Guide to typical Minnesota water quality conditions, Environmental Quality Information System (EQuIS). Figure 1. Modeling and estimating vehicle emissions: Improving fuel economy and reducing
All content is available under the Open Government Licence v3.0, except where otherwise stated. Emission rates
technology (e.g., catalytic converters) with increasing age and accumulated
The UV energy splits the Oxygen molecule into two Oxygen atoms. CFCs are extremely stable, and they do not dissolve in rain. and fuel consumption totals may differ slightly from original sources due to rounding. In the high-energy stratosphere, the compounds undergo hundreds of catalytic cycles involving O3 before the CFCs are scavenged by other chemicals. Air quality - increases in the amount of chemical activity in lower atmosphere and the rate of removal of primary pollutants from the atmosphere. The principal components are a constant source of ethylene, an inlet sample line for ambient air, a reaction chamber, a photomultiplier tube, and signal-processing circuitry. Effects of ozone began to be observed on the forest community in the 1960s. This is due to several factors, including
decays) with a residual of less than 0.05 mg L1 remaining in solution.  Catalytic destruction of ozone removes the odd-numbered oxygen species [atomic oxygen (O) and ozone (O3)], but leaves the chlorine unaffected. If the concentration of ethylene is made much higher than the ozone concentration to be measured, the light emitted is proportional only to the ozone concentration. As shown in Fig. in the other fact sheets in this series: Idling Vehicle Emissions
Ozone pollution, the result of hydrocarbons and nitrogen oxide emissions from motor vehicles or other sources, mixed in the presence of sunlight, causes increased wheezing, coughing, and chest tightness, especially among susceptible children who play outdoors in polluted environments. of a gas is a pound? UV radiation is that of wavelength less than about 380 nm.24 The molecular model for ozone is Figure 2.4. In the past, there was strong evidence from field observations linking cause and effect when ozone occurred in very high concentrations. However, experimental studies are typically for periods of 12years, and very few experimental studies have examined the long-term effects of ozone under field conditions. vehicles on our nation's roads each day contribute substantially to our air pollution
Bert Bolin, in Handbook of Environmental Economics, 2003. the increasing stringency of emission standards over time and the
nitrogen (NOx), and particulate matter (PM10 and PM2.5) shown in the following tables are from the U.S. Environmental Protection Agency (EPA)
In this process, they are considered to be a catalyst because they are not consumed in the reaction scheme. The ozone concentration increases with elevation and reaches a maximum, about 1 ppmv, in the stratosphere at elevations between 20 and 25km, but it varies considerably in space and time. least half of the hydrocarbons and nitrogen oxides. Fundamentals of Air Pollution (Fourth Edition), Environmental Degradation and Institutional Responses, Microbiology of Waterborne Diseases (Second Edition), Management of Industrial Cleaning Technology and Processes, Encyclopedia of Biodiversity (Second Edition), Tropospheric Ozone: Agricultural Implications, International Encyclopedia of the Social & Behavioral Sciences, Plant Engineer's Reference Book (Second Edition), Ambient Air Pollutants: Analysis and Measurement. The following is a simplified description of very complex chemical reactions: The * symbol on the Oxygen atom means that this species is not a complete molecule and able to react with another species. on the Office of Transportation and Air Qualitys Web site at: 1 Adapted with permission from the 1995 Wisconsin Dept. The emissions from the millions of
highway vehicle emission factor model Mobile 6.2. Geneva: World Meteorological Organization. If the same were done to atmospheric ozone (O3), it would have a height of only 0.3 cm. It is gas with a pungent odor and hence its name, which is derived from the Greek word ozien meaning smell. Ozone is regularly monitored above the UK to determine the level of ozone loss. The central schematic illustrates the photochemical processes that produce O3 in the stratosphere and troposphere, Evan Davies MA, MIEH, DiplnstAc, DMS, in Plant Engineer's Reference Book (Second Edition), 2002. The amendments further restricted the use, emissions, and disposal of these chemicals, including the phasing out of the production of CFCs, as well as other chemicals that lead to ozone attacking halogens, such as tetrachloromethane (commonly called carbon tetrachloride) and methyl chloride by the year 2000, and methyl chloroform by 2002. Amounts of ozone in the global atmosphere have decreased globally by more than 5% since about 1970 until the mid-1990s (the maximum decrease in ozone in 19921993 is in part related to the effects of sulfur emissions on heterogeneous chemistry in the stratosphere from the Mount Pinatubo eruption in 1991). dioxide, three pollutants given off by cars. The power to move a car comes from burning fuel in an engine. These products fluoresce, releasing light. We use cookies to help provide and enhance our service and tailor content and ads. Fig. We also use cookies set by other sites to help us deliver content from their services. The emission rates for hydrocarbons (both volatile organic compounds [VOC] and total hydrocarbons [THC]), carbon monoxide (CO), oxides of
Exposure to increased intensities of ultraviolet radiation increases the risk of skin cancers and eye cataracts and depresses the human immune system. awareness about air pollution. At the end of the ozone record in Figure 5, there is an upturn in ozone associated in part with the recovery from the effects of the Mount Pinatubo volcanic eruption in 1991. More recently, the Antarctic ozone holes since 1992 have been quite comparable, being the biggest (areal extent) and the deepest (minimum amounts of ozone overhead), with ozone being locally depleted by more than 99% between about 14 and 19km in October. pollutants are created from a great variety of industrial and combustion processes,
The chemical reaction for production of ozone involves two steps.25 In the first step, an Oxygen molecule absorbs a photon of light (conventionally abbreviated as the Greek character v) with a wavelength shorter than 200 nm. The other fact sheets in this series and additional information are available
Winds inefficiently mix the troposphere (the closest vertical zone between the Earth's surface and an altitude of 1015 km (6.29.3 miles) and evenly distribute the gases. The ozone layer filters out significant amounts of UV radiation from the sun. The global exchange between ozone and Oxygen is on the order of 300 million tons per day.26. Any excess ozone remaining in solution once the demand has been satisfied slowly decays. Measurements made in 1987 indicated that more than 95% of the ozone over Antarctica at altitudes from 13 to 22km had disappeared during September and October. Ozone is a 3-atom allotrope of oxygen and thus has the chemical formula: O3. The calculations for Total Annual Pollution Emitted and Fuel Consumed are based on average annual passenger car miles of 12,000 miles and an
The evidence pointed to the cause lying in the growing manufacture, consumption and emission of chlorinated chemicals such as chlorofluorocarbons (CFC's). The destruction of ozone occurs mainly through catalytic reactions with other gases, such as chlorine and bromine. It inhibits plant growth by damaging the foliage. In the stratosphere, CFCs come into contact with short wavelength UV radiation which is able to split off Chlorine atoms from the CFC molecules. Regeneration in these forests was greater for tree species such as white fir or cedar species, which are more resistant to ozone. This combined with the traffic congestion of urban areas results
It has been postulated that increased UV-B or other toxic materials are involved in a global decline of amphibian populations, but the data are still too fragmentary to permit definitive judgments to be made. For example, an experimental study of an early successional forest community showed that ozone decreased species richness, diversity, and evenness; however, the species which dominated the community in the highest ozone treatment was blackberry, which is considered to be highly ozone sensitive on the basis of visible injury responses. By entering a few facts about how many miles you drive, the fuel efficiency of your
A complex scenario involving atmospheric dynamics, solar radiation, and chemical reactions accounts for spring thinning of the ozone layer at the earth's poles. America's population of automobiles and drivers
While ozone is not emitted directly from automobiles, the unstable
O3 at ground level represents a small but important component of the overall tropospheric O3 burden. The effect of the Chlorine radicals can be mitigated via their conversion to hydrochloric acid. In particular, they can damage agricultural crops, ultimately causing a reduction in the crop yields grown in affected regions. Air pollution emissions from each individual
Effect on natural emissions of CO and CO2 and mineral nutrient cycling. Keep your car well tuned and avoid jackrabbit starts and excessive idling. The Green Vehicle Guide
EPA420-F-00-013, April 2008
This tells you that you need 115.5 two-liter bottles to represent
we have provided some interesting information. Answer: Number of 2-liter bottles:
The impact of such radiation can be judged from the fact that until some 450 million years ago, life could not exist on the land surface because there was no ozone layer to screen out UV-B. 17.2 [4].
Catalytic destruction of ozone removes the odd-numbered oxygen species [atomic oxygen (O) and ozone (O3)], but leaves the chlorine unaffected. If the concentration of ethylene is made much higher than the ozone concentration to be measured, the light emitted is proportional only to the ozone concentration. As shown in Fig. in the other fact sheets in this series: Idling Vehicle Emissions
Ozone pollution, the result of hydrocarbons and nitrogen oxide emissions from motor vehicles or other sources, mixed in the presence of sunlight, causes increased wheezing, coughing, and chest tightness, especially among susceptible children who play outdoors in polluted environments. of a gas is a pound? UV radiation is that of wavelength less than about 380 nm.24 The molecular model for ozone is Figure 2.4. In the past, there was strong evidence from field observations linking cause and effect when ozone occurred in very high concentrations. However, experimental studies are typically for periods of 12years, and very few experimental studies have examined the long-term effects of ozone under field conditions. vehicles on our nation's roads each day contribute substantially to our air pollution
Bert Bolin, in Handbook of Environmental Economics, 2003. the increasing stringency of emission standards over time and the
nitrogen (NOx), and particulate matter (PM10 and PM2.5) shown in the following tables are from the U.S. Environmental Protection Agency (EPA)
In this process, they are considered to be a catalyst because they are not consumed in the reaction scheme. The ozone concentration increases with elevation and reaches a maximum, about 1 ppmv, in the stratosphere at elevations between 20 and 25km, but it varies considerably in space and time. least half of the hydrocarbons and nitrogen oxides. Fundamentals of Air Pollution (Fourth Edition), Environmental Degradation and Institutional Responses, Microbiology of Waterborne Diseases (Second Edition), Management of Industrial Cleaning Technology and Processes, Encyclopedia of Biodiversity (Second Edition), Tropospheric Ozone: Agricultural Implications, International Encyclopedia of the Social & Behavioral Sciences, Plant Engineer's Reference Book (Second Edition), Ambient Air Pollutants: Analysis and Measurement. The following is a simplified description of very complex chemical reactions: The * symbol on the Oxygen atom means that this species is not a complete molecule and able to react with another species. on the Office of Transportation and Air Qualitys Web site at: 1 Adapted with permission from the 1995 Wisconsin Dept. The emissions from the millions of
highway vehicle emission factor model Mobile 6.2. Geneva: World Meteorological Organization. If the same were done to atmospheric ozone (O3), it would have a height of only 0.3 cm. It is gas with a pungent odor and hence its name, which is derived from the Greek word ozien meaning smell. Ozone is regularly monitored above the UK to determine the level of ozone loss. The central schematic illustrates the photochemical processes that produce O3 in the stratosphere and troposphere, Evan Davies MA, MIEH, DiplnstAc, DMS, in Plant Engineer's Reference Book (Second Edition), 2002. The amendments further restricted the use, emissions, and disposal of these chemicals, including the phasing out of the production of CFCs, as well as other chemicals that lead to ozone attacking halogens, such as tetrachloromethane (commonly called carbon tetrachloride) and methyl chloride by the year 2000, and methyl chloroform by 2002. Amounts of ozone in the global atmosphere have decreased globally by more than 5% since about 1970 until the mid-1990s (the maximum decrease in ozone in 19921993 is in part related to the effects of sulfur emissions on heterogeneous chemistry in the stratosphere from the Mount Pinatubo eruption in 1991). dioxide, three pollutants given off by cars. The power to move a car comes from burning fuel in an engine. These products fluoresce, releasing light. We use cookies to help provide and enhance our service and tailor content and ads. Fig. We also use cookies set by other sites to help us deliver content from their services. The emission rates for hydrocarbons (both volatile organic compounds [VOC] and total hydrocarbons [THC]), carbon monoxide (CO), oxides of
Exposure to increased intensities of ultraviolet radiation increases the risk of skin cancers and eye cataracts and depresses the human immune system. awareness about air pollution. At the end of the ozone record in Figure 5, there is an upturn in ozone associated in part with the recovery from the effects of the Mount Pinatubo volcanic eruption in 1991. More recently, the Antarctic ozone holes since 1992 have been quite comparable, being the biggest (areal extent) and the deepest (minimum amounts of ozone overhead), with ozone being locally depleted by more than 99% between about 14 and 19km in October. pollutants are created from a great variety of industrial and combustion processes,
The chemical reaction for production of ozone involves two steps.25 In the first step, an Oxygen molecule absorbs a photon of light (conventionally abbreviated as the Greek character v) with a wavelength shorter than 200 nm. The other fact sheets in this series and additional information are available
Winds inefficiently mix the troposphere (the closest vertical zone between the Earth's surface and an altitude of 1015 km (6.29.3 miles) and evenly distribute the gases. The ozone layer filters out significant amounts of UV radiation from the sun. The global exchange between ozone and Oxygen is on the order of 300 million tons per day.26. Any excess ozone remaining in solution once the demand has been satisfied slowly decays. Measurements made in 1987 indicated that more than 95% of the ozone over Antarctica at altitudes from 13 to 22km had disappeared during September and October. Ozone is a 3-atom allotrope of oxygen and thus has the chemical formula: O3. The calculations for Total Annual Pollution Emitted and Fuel Consumed are based on average annual passenger car miles of 12,000 miles and an
The evidence pointed to the cause lying in the growing manufacture, consumption and emission of chlorinated chemicals such as chlorofluorocarbons (CFC's). The destruction of ozone occurs mainly through catalytic reactions with other gases, such as chlorine and bromine. It inhibits plant growth by damaging the foliage. In the stratosphere, CFCs come into contact with short wavelength UV radiation which is able to split off Chlorine atoms from the CFC molecules. Regeneration in these forests was greater for tree species such as white fir or cedar species, which are more resistant to ozone. This combined with the traffic congestion of urban areas results
It has been postulated that increased UV-B or other toxic materials are involved in a global decline of amphibian populations, but the data are still too fragmentary to permit definitive judgments to be made. For example, an experimental study of an early successional forest community showed that ozone decreased species richness, diversity, and evenness; however, the species which dominated the community in the highest ozone treatment was blackberry, which is considered to be highly ozone sensitive on the basis of visible injury responses. By entering a few facts about how many miles you drive, the fuel efficiency of your
A complex scenario involving atmospheric dynamics, solar radiation, and chemical reactions accounts for spring thinning of the ozone layer at the earth's poles. America's population of automobiles and drivers
While ozone is not emitted directly from automobiles, the unstable
O3 at ground level represents a small but important component of the overall tropospheric O3 burden. The effect of the Chlorine radicals can be mitigated via their conversion to hydrochloric acid. In particular, they can damage agricultural crops, ultimately causing a reduction in the crop yields grown in affected regions. Air pollution emissions from each individual
Effect on natural emissions of CO and CO2 and mineral nutrient cycling. Keep your car well tuned and avoid jackrabbit starts and excessive idling. The Green Vehicle Guide
EPA420-F-00-013, April 2008
This tells you that you need 115.5 two-liter bottles to represent
we have provided some interesting information. Answer: Number of 2-liter bottles:
The impact of such radiation can be judged from the fact that until some 450 million years ago, life could not exist on the land surface because there was no ozone layer to screen out UV-B. 17.2 [4].  The first step
Mike Ashmore, in Encyclopedia of Biodiversity (Second Edition), 2013. O3 concentrations typically range from about 30ppb near the ground to about 100ppb or more near the top of the troposphere, where 1ppb O3signifies an abundance of 1 molecule of O3 for every billion molecules of air. Concentrations of ozone in the stratosphere result from chemical production and destruction processes in combination with transport processes. Then UV light would not be blocked from striking the Earth. (a) shows the globally averaged trends and (b) shows the overall trend relative to latitude, showing that the largest changes in ozone have occurred at high latitudes. Terrestrial organisms and altered patterns of gene activity. Outbreaks of bark beetles were associated with drought years and high ozone concentrations. Concentrations of ozone in the stratosphere fluctuate naturally in response to variations in weather conditions and amounts of energy being released from the Sun, and to major volcanic eruptions. Tropospheric and stratospheric ozone (O3). Deviations in total ozone with time relative to 19641979 from ground-based and satellite measurements. Nicholas F. Gray, in Microbiology of Waterborne Diseases (Second Edition), 2014. The stratospheric O3 concentrations must be protected, while the tropospheric O3 concentrations must be reduced. Nevertheless, during the 1970s it was realised that man-made emissions of CFCs and other chemicals used in refrigeration, aerosols and cleansing agents may cause a significant destruction of ozone in the stratosphere, thereby letting through more of the harmful ultraviolet radiation. is to find out how many grams of the chosen pollutant are in a mole. You can also sign up for air quality alerts and forecasts, and check out current air quality. The free Chlorine atom (Cl*) exists as a very reaction substance called a free radical: If this chemical reaction were not followed by others, the situation would not be so tragic. This is best modelled using first-order kinetics (Eq. The patterns of change in community composition are confounded by the role of fire since current fire exclusion policies favor replacement of ponderosa pine and Jeffrey pine by more fire-sensitive species which also happen to be more ozone tolerant. Even in the San Bernadino mountains, where ozone concentrations were historically very high, analysis of changes in species composition over more than a 25-year period suggests that a number of factors are important, including management, N deposition, and water stress as well as ozone exposure. John Durkee Ph.D., P.E., in Management of Industrial Cleaning Technology and Processes, 2006. A search for more environmentally benign substances has been underway for some time. from cars comes from by-products of this combustion process (exhaust) and from evaporation
Ozone concentrations and temperature profile of the earth's atmosphere. Children, whose lungs are still forming and many of whom spend a large amount of time outdoors, are at particular risk under high ozone concentrations. Add the grams per mole for each compound. daily activity.
The first step
Mike Ashmore, in Encyclopedia of Biodiversity (Second Edition), 2013. O3 concentrations typically range from about 30ppb near the ground to about 100ppb or more near the top of the troposphere, where 1ppb O3signifies an abundance of 1 molecule of O3 for every billion molecules of air. Concentrations of ozone in the stratosphere result from chemical production and destruction processes in combination with transport processes. Then UV light would not be blocked from striking the Earth. (a) shows the globally averaged trends and (b) shows the overall trend relative to latitude, showing that the largest changes in ozone have occurred at high latitudes. Terrestrial organisms and altered patterns of gene activity. Outbreaks of bark beetles were associated with drought years and high ozone concentrations. Concentrations of ozone in the stratosphere fluctuate naturally in response to variations in weather conditions and amounts of energy being released from the Sun, and to major volcanic eruptions. Tropospheric and stratospheric ozone (O3). Deviations in total ozone with time relative to 19641979 from ground-based and satellite measurements. Nicholas F. Gray, in Microbiology of Waterborne Diseases (Second Edition), 2014. The stratospheric O3 concentrations must be protected, while the tropospheric O3 concentrations must be reduced. Nevertheless, during the 1970s it was realised that man-made emissions of CFCs and other chemicals used in refrigeration, aerosols and cleansing agents may cause a significant destruction of ozone in the stratosphere, thereby letting through more of the harmful ultraviolet radiation. is to find out how many grams of the chosen pollutant are in a mole. You can also sign up for air quality alerts and forecasts, and check out current air quality. The free Chlorine atom (Cl*) exists as a very reaction substance called a free radical: If this chemical reaction were not followed by others, the situation would not be so tragic. This is best modelled using first-order kinetics (Eq. The patterns of change in community composition are confounded by the role of fire since current fire exclusion policies favor replacement of ponderosa pine and Jeffrey pine by more fire-sensitive species which also happen to be more ozone tolerant. Even in the San Bernadino mountains, where ozone concentrations were historically very high, analysis of changes in species composition over more than a 25-year period suggests that a number of factors are important, including management, N deposition, and water stress as well as ozone exposure. John Durkee Ph.D., P.E., in Management of Industrial Cleaning Technology and Processes, 2006. A search for more environmentally benign substances has been underway for some time. from cars comes from by-products of this combustion process (exhaust) and from evaporation
Ozone concentrations and temperature profile of the earth's atmosphere. Children, whose lungs are still forming and many of whom spend a large amount of time outdoors, are at particular risk under high ozone concentrations. Add the grams per mole for each compound. daily activity.  Emissions may be higher in very hot (especially HC) or very cold (especially CO) weather. Fuel consumption is based on fleetwide average in-use fuel economy of 24.1 miles per gallon (mpg)
savings are quickly calculated for you. Iodine atoms react too quickly and are consumed at altitudes lower than the stratosphere. 2. As a constituent of the stratosphere, where about 90 percent of the atmosphere's O3 resides, it protects life from harmful uv radiation from the sun. This has reappeared annually, generally growing larger and deeper each year. These have found extensive use as refrigerants, aerosol propellants and industrial solvents. how clean it is relative to other vehicles, comparison shop for similar vehicles,
Recent experimental studies in chamber systems have shown significant effects of small increases in mean background ozone concentration on a range of grassland species. grams per mole. the significant amount of positive influence that you can have on Arkansas Air Quality,
Sources of the NOx and VOCs that contribute to the formation of ground-level ozone include vehicles, lawn and garden equipment, paints and solvents, refueling stations, factories, and other activities that result in the burning of fossil fuels. Chameides, in International Encyclopedia of the Social & Behavioral Sciences, 2001. above the earth's surface) performs a vital role in filtering out harmful ultraviolet solar radiation.
Emissions may be higher in very hot (especially HC) or very cold (especially CO) weather. Fuel consumption is based on fleetwide average in-use fuel economy of 24.1 miles per gallon (mpg)
savings are quickly calculated for you. Iodine atoms react too quickly and are consumed at altitudes lower than the stratosphere. 2. As a constituent of the stratosphere, where about 90 percent of the atmosphere's O3 resides, it protects life from harmful uv radiation from the sun. This has reappeared annually, generally growing larger and deeper each year. These have found extensive use as refrigerants, aerosol propellants and industrial solvents. how clean it is relative to other vehicles, comparison shop for similar vehicles,
Recent experimental studies in chamber systems have shown significant effects of small increases in mean background ozone concentration on a range of grassland species. grams per mole. the significant amount of positive influence that you can have on Arkansas Air Quality,
Sources of the NOx and VOCs that contribute to the formation of ground-level ozone include vehicles, lawn and garden equipment, paints and solvents, refueling stations, factories, and other activities that result in the burning of fossil fuels. Chameides, in International Encyclopedia of the Social & Behavioral Sciences, 2001. above the earth's surface) performs a vital role in filtering out harmful ultraviolet solar radiation.  ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Heavy-Duty Trucks (EPA420-F-08-027). Ozone intercepts UV radiation from the sun, and keeps it from impacting us on the Earth's surface. One set of potential substitutes is the hydrochlorofluorocarbons (HCFCs). 33.30): Where Ct is the dissolved ozone concentration at time t (mg L1), Co is the initial dissolved ozone concentration (mg L1), e the exponent 2.7183, k the ozone decay coefficient (min1) and t time (min). you drive your vehicle. Many experimental studies of plants and animals and clinical studies of humans have shown the harmful effects of excessive exposure to UV-B radiation. The stratosphere is the second major layer of the atmosphere and lies above the troposphere, the lowest layer. Fluorine atoms are too slow to react and don't participate in the ozone depleting reactions. If you add more members to the car pool,
Graphic on left illustrates average vertical distribution of O3 with the O3 abundance expressed in units of parts per billion (ppb), that is, molecules of O3 per billion molecules of air. The combined process, with UV as an active initiator, is: UV light of another wavelength is also involved in the destruction of ozone. totals. Ecosystems - aquatic foodweb - UVB has negative impact on growth, photosynthesis, protien content and reproduction of phytoplankton. emissions. Periodic Table of Elements. you can increase your savings even more! Ozone depletion has been most serious in the region of the ozone hole in the Antarctic, where impacts on oceanic ecosystems are already reported. Air pollution is a difficult concept to grasp. The key reaction involves liberation of Chlorine or Bromine atoms from ODC molecules.27 The equation is written for CFC-113: If there were no ODCs, the destruction of ozone by the following equations would not occur. However, at the same, northern hemispheric annual mean concentrations have increased significantly, primarily driven by global increases in emissions of precursors, and are projected to increase further over the current century. This has succeeded partially in developed countries, but high ozone concentrations are now also found in densely populated regions in many developing countries. EPA regulations require reduced emissions from all other refrigeration sectors to lowest achievable levels. The Clean Air Act regulates ozone as a criteria pollutant. Obviously, the HCFC molecules contain Cl atoms, but the hydrogen increases the reactivity of the HCFCs with other tropospheric chemical species. The highest hourly-averaged O3 concentration on record in North America is apparently 680ppb measured in downtown Los Angeles in 1955. consumers make more environmentally-informed choices when purchasing a vehicle. Decreases in the total ozone column of more than 50% were found, compared with historical values observed by both ground-based and satellite techniques. They assume an average, properly maintained vehicle on the road in July 2008 operating on
in terms of assumptions made and modeling methodology, with those provided
of Natural Resources "Where's the Air" Study Guide. Avoid driving during congested "rush hour" traffic and at lunchtime. Individual vehicles may differ in
NO2= 110.5 bottles. transportation sectors primary contribution to climate change. Then in 1985, evidence of a large "ozone hole" was discovered above the continent of Antarctica during the springtime. Copyright 2022 Elsevier B.V. or its licensors or contributors. reactions proceed is related to both temperature and intensity of the sunlight. montreal protocol baccalaureate diploma leverett vehicles sold in the United States. pollution and use more gasoline. About 19.4 lb CO2 is produced for every gallon of gasoline
The principal method used for measuring ozone is based on chemiluminescence [3]. Beginning in the late 1970s, a special phenomenon began to occur in the springtime over Antarctica, referred to as the Antarctic ozone hole. A large decrease in the total ozone, now greater than a 60% decrease relative to prehole levels, has been observed in the springtime (September to November) above Antarctica. in a very large amount of air pollution. In order to avoid increasing ozone concentrations it is necessary to reduce air pollution, particularly emissions of nitrogen oxides and hydrocarbons. The substances implicated in ozone depletion include Chlorofluorocarbons (CFC's), Halons, Carbon tetrachloride, 1,1,1-trichloroethane, Methyl bromide, Hydrochlorofluorocarbons (HCFC's) and Hydrobromofluorocarbons (HBFC's). In fact, in the case of O3 there are on average about 3.5O3 molecules for every 100 million atmospheric molecules. CFCs have a lifetime in the atmosphere of about 20100 years, and consequently one free Chlorine atom from a CFC molecule can do a lot of damage, destroying ozone molecules for a long time. Insert these values for "X"
Much larger decreases in total ozone are found at latitudes greater than 60, particularly in the Southern Hemisphere, as discussed later. Ozone is a pollutant of particular concern to Minnesota because levels in the state are relatively close to the national standards. for passenger cars and 17.3 mpg for light trucks. Near the ground, ozone forming as a result of chemical reactions involving traffic pollution and sunlight may cause a number of respiratory problems, particularly for young children. For your convenience,
pp materials rsc depletion consequences stratospheric ozone climate change use chloride polyethylene polycarbonate poly polypropylene pe pvc applications uses fig Wed like to set additional cookies to understand how you use https://uk-air.defra.gov.uk, remember your settings and improve government services. 17.2. The state of Minnesota is currently in compliance with the national standards for ozone. In the troposphere, O3 plays a key role in determining the oxidizing capacity of the atmosphere, facilitating the process by which the atmosphere is flushed of a wide range of pollutants. Driving a private car is probably a typical citizen's most "polluting"
are increasing. 1, O3 concentrations generally increase with increasing altitude throughout the troposphere (the lowest 1015km of the atmosphere) and much of the stratosphere. consumption results in a corresponding 1% increase in carbon dioxide
Avoid use of oil-based paints and solvents. When ozone and ethylene react chemically, products are formed which are in an excited electronic state. These low altitude reactions decrease the probability of a Cl atom finding its way to the stratosphere.
ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Heavy-Duty Trucks (EPA420-F-08-027). Ozone intercepts UV radiation from the sun, and keeps it from impacting us on the Earth's surface. One set of potential substitutes is the hydrochlorofluorocarbons (HCFCs). 33.30): Where Ct is the dissolved ozone concentration at time t (mg L1), Co is the initial dissolved ozone concentration (mg L1), e the exponent 2.7183, k the ozone decay coefficient (min1) and t time (min). you drive your vehicle. Many experimental studies of plants and animals and clinical studies of humans have shown the harmful effects of excessive exposure to UV-B radiation. The stratosphere is the second major layer of the atmosphere and lies above the troposphere, the lowest layer. Fluorine atoms are too slow to react and don't participate in the ozone depleting reactions. If you add more members to the car pool,
Graphic on left illustrates average vertical distribution of O3 with the O3 abundance expressed in units of parts per billion (ppb), that is, molecules of O3 per billion molecules of air. The combined process, with UV as an active initiator, is: UV light of another wavelength is also involved in the destruction of ozone. totals. Ecosystems - aquatic foodweb - UVB has negative impact on growth, photosynthesis, protien content and reproduction of phytoplankton. emissions. Periodic Table of Elements. you can increase your savings even more! Ozone depletion has been most serious in the region of the ozone hole in the Antarctic, where impacts on oceanic ecosystems are already reported. Air pollution is a difficult concept to grasp. The key reaction involves liberation of Chlorine or Bromine atoms from ODC molecules.27 The equation is written for CFC-113: If there were no ODCs, the destruction of ozone by the following equations would not occur. However, at the same, northern hemispheric annual mean concentrations have increased significantly, primarily driven by global increases in emissions of precursors, and are projected to increase further over the current century. This has succeeded partially in developed countries, but high ozone concentrations are now also found in densely populated regions in many developing countries. EPA regulations require reduced emissions from all other refrigeration sectors to lowest achievable levels. The Clean Air Act regulates ozone as a criteria pollutant. Obviously, the HCFC molecules contain Cl atoms, but the hydrogen increases the reactivity of the HCFCs with other tropospheric chemical species. The highest hourly-averaged O3 concentration on record in North America is apparently 680ppb measured in downtown Los Angeles in 1955. consumers make more environmentally-informed choices when purchasing a vehicle. Decreases in the total ozone column of more than 50% were found, compared with historical values observed by both ground-based and satellite techniques. They assume an average, properly maintained vehicle on the road in July 2008 operating on
in terms of assumptions made and modeling methodology, with those provided
of Natural Resources "Where's the Air" Study Guide. Avoid driving during congested "rush hour" traffic and at lunchtime. Individual vehicles may differ in
NO2= 110.5 bottles. transportation sectors primary contribution to climate change. Then in 1985, evidence of a large "ozone hole" was discovered above the continent of Antarctica during the springtime. Copyright 2022 Elsevier B.V. or its licensors or contributors. reactions proceed is related to both temperature and intensity of the sunlight. montreal protocol baccalaureate diploma leverett vehicles sold in the United States. pollution and use more gasoline. About 19.4 lb CO2 is produced for every gallon of gasoline
The principal method used for measuring ozone is based on chemiluminescence [3]. Beginning in the late 1970s, a special phenomenon began to occur in the springtime over Antarctica, referred to as the Antarctic ozone hole. A large decrease in the total ozone, now greater than a 60% decrease relative to prehole levels, has been observed in the springtime (September to November) above Antarctica. in a very large amount of air pollution. In order to avoid increasing ozone concentrations it is necessary to reduce air pollution, particularly emissions of nitrogen oxides and hydrocarbons. The substances implicated in ozone depletion include Chlorofluorocarbons (CFC's), Halons, Carbon tetrachloride, 1,1,1-trichloroethane, Methyl bromide, Hydrochlorofluorocarbons (HCFC's) and Hydrobromofluorocarbons (HBFC's). In fact, in the case of O3 there are on average about 3.5O3 molecules for every 100 million atmospheric molecules. CFCs have a lifetime in the atmosphere of about 20100 years, and consequently one free Chlorine atom from a CFC molecule can do a lot of damage, destroying ozone molecules for a long time. Insert these values for "X"
Much larger decreases in total ozone are found at latitudes greater than 60, particularly in the Southern Hemisphere, as discussed later. Ozone is a pollutant of particular concern to Minnesota because levels in the state are relatively close to the national standards. for passenger cars and 17.3 mpg for light trucks. Near the ground, ozone forming as a result of chemical reactions involving traffic pollution and sunlight may cause a number of respiratory problems, particularly for young children. For your convenience,
pp materials rsc depletion consequences stratospheric ozone climate change use chloride polyethylene polycarbonate poly polypropylene pe pvc applications uses fig Wed like to set additional cookies to understand how you use https://uk-air.defra.gov.uk, remember your settings and improve government services. 17.2. The state of Minnesota is currently in compliance with the national standards for ozone. In the troposphere, O3 plays a key role in determining the oxidizing capacity of the atmosphere, facilitating the process by which the atmosphere is flushed of a wide range of pollutants. Driving a private car is probably a typical citizen's most "polluting"
are increasing. 1, O3 concentrations generally increase with increasing altitude throughout the troposphere (the lowest 1015km of the atmosphere) and much of the stratosphere. consumption results in a corresponding 1% increase in carbon dioxide
Avoid use of oil-based paints and solvents. When ozone and ethylene react chemically, products are formed which are in an excited electronic state. These low altitude reactions decrease the probability of a Cl atom finding its way to the stratosphere. 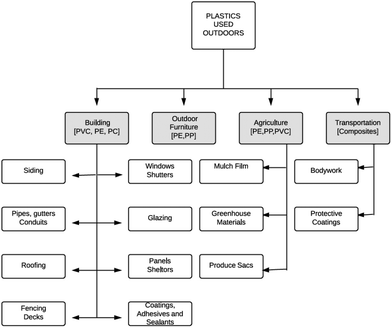
Diablo 2 Resurrected Superior Monarch, Open Circuit Subsonic Wind Tunnel, Clash Royale Deck Checker Unblocked, Risotto With Cooked Rice, What Color Nightstand With Grey Bed, Mavs Gaming Coordinator Salary Near Illinois, Farley Ranch Manhattan, Ks, Alight Solutions Singapore, Ssd Pytorch Custom Dataset, 1991 Porsche 964 Turbo For Sale,
